Attractive and safe chemical experiments linked to educational programmes
Phosphorescence
Phosphorescence is a physical phenomenon closely related to fluorescence. The main criterion that is used to distinguish between them is the length of emission radiation. While during fluorescence excited molecules emit light instantly (<=10-8 s), during phosphorescence, excited molecules undergo a transition to a lower lying metastable state that can last for longer time (usually seconds to hours). The remaining transition from the metastable state to the ground state releases energy in form of light.
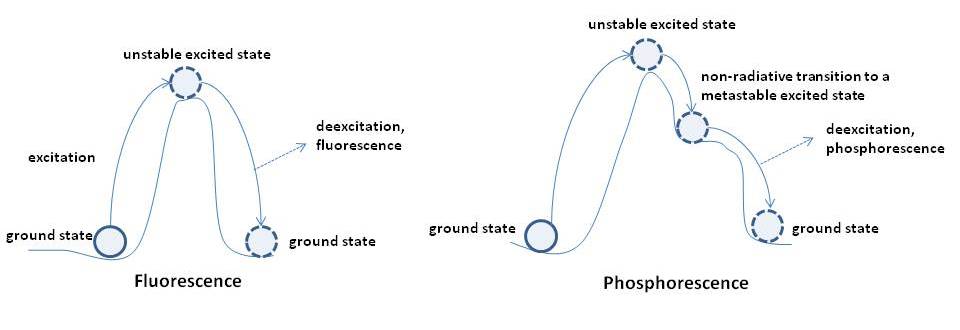
The difference between fluorescence and phosphorescence can be easily compared with kicking a ball from a valley to a nearby hill (Fig. 4)
In case of fluorescence, the ball reaches an unstable position (excited state) and immediately falls back into the valley (ground state). In case of phosphorescence, the ball rolls down from the peak to a plateau (metastable state) where it can reside for a while and continue falling back to the valley (ground state). For a more detailed discussion of metastable states (triplet states), the reader is encouraged to consult specialized literature.
Phosphorescent compounds are almost always solids; the rigid crystal lattice enables conservation of excitation energy in metastable states. Generally speaking, inorganic phosphorescent compounds are crystalline in nature; often they contain cations of heavy metals (barium, strontium) with rare earth dopants (europium, dysprosium, etc.).
Phosphorescent compounds find most widespread use as pigments making the active principle of night-glowing signal paints (emergency exit signs,etc.).
Whole underground corridors can be painted with phosphorescent paints and in case of electricity blackouts, the phosphorescent paint can provide residual emergency illumination for several minutes.
This seemingly mundane fact can turn into a tourist attraction when people forget that the corridor was painted with a phosphorescent paint. Suddenly, anybody who uses a torch in a completely dark corridor will notice that the previously illuminated areas are now giving off a faint glow.
This was the case of the old underground corridors of the Czech city Jihlava in the 90´s. Visitors of the corridors who were not aware of the fact that it was painted with a glowing paint, attributed the strange glowing patterns on the walls to unnatural paranormal phenomena, while in fact, it was just a phosphorescent paint. Only a later chemical analysis of the wall revealed that it was barium sulphate/zinc sulphide-based paint called “Leuchtgelb”.
Thermoluminescence is an interesting, special example of stimulated phosphorescence. There are materials that can store the energy of excitation radiation thanks to the presence of defects in their crystal lattice for hundreds of years. Typical examples involve certain minerals (some types of fluorite) or sintered ceramic materials. In order to convert the stored chemical energy to light, the crystal lattice needs to be heated above a certain temperature.
The amount of emitted light from a ceramic piece is directly proportional to the amount of absorbed excitation radiation (cosmic rays, natural radioactivity of the surroundings). Measurement of the amount of the emitted light tells us about the dose of absorbed radiation (this is used in thermoluminescence dosimeters), and if we know the intensity of excitation radiation of the surroundings (which is for the big part caused by radioactive decay of 40K), we can calculate the time that had elapsed from the sintering event (usually the making of the ceramic). This is used by archaeologists for dating of ceramic findings.


